Radioactive Isotopes
Those radioactive isotopes that emit X-rays during their decay can be used as X-ray sources. One of the types of decay is the so-called internal transition (IT), during which the energy released is not carried away from the atomic nucleus by an emitted gamma photon, but by one of the inner-shell electrons as it leaves the bond. The state of electron deficiency formed in this way (vacancy) is filled by an electron from a higher energy level; the atom returns to a lower energy state by the emission of a characteristic X-ray photon. Internal transition usually “competes with” the gamma radiation, primarily in transitions when the probability of a gamma transition is forbidden in first order by some sort of hindering factor (e.g. a large change in angular momentum). Another possibility in the case of nuclei having a “proton excess” as compared to other isotopes is electron capture (EC), during which one of the inner-shell electrons is captured by the nucleus. The electron combines with a proton and forms a neutron, thus stabilizing the nucleus.
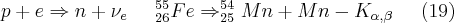
The vacancy formed in the place of the captured electron can be filled by an electron from a higher-energy shell. During the process an X-ray photon which is characteristic of the daughter element is emitted; the energy of this photon is dependent on the electron transition that is realized out of the possible electron transitions of the daughter element.
Similarly to the de-excitations occurring in the photoelectric effect, the creation of the individual X-ray transitions is determined by their transition probabilities, as a result of which the ratios of the intensities of the individual characteristic X-ray lines have specific values in the detected X-ray spectra.
Table 2. Various radioisotopes
| The most frequently used radioisotopes | Half-life | Emitted X-ray line (keV) | Yield per decay (%) | Type of decay |
| 55Fe | 2.7 years | 5.9 (Mn-Kα) | 26 | electron capture |
| 109Cd | 453 days | 22.1 (Ag-Kα), 87.7 (γ) | 107, 4 | electron capture |
| 241Am | 458 years | 59.6 (γ), 26.4, (γ) | 36, 40 | alpha radiation |
| 57Co | 270 days | 136 (γ), 122 (γ), 14.4 (γ) | 85.2, 9.7, 51 | electron capture |
Radioactive materials as X-ray sources are usually used in X-ray fluorescence analysis, when the daughter element, which is formed after the decay, emits the radiation characteristic of it. As compared to the X-ray tubes, this type of radiation source has favourable features: it is compact, portable, it can be produced in small sizes, and only a few discrete energy values appear in its spectrum (the characteristic X-ray lines of the daughter element). The secondary X-ray spectrum formed during the excitation is free of the intense Bremsstrahlung spectrum, which is observable in the spectra of X-ray tubes. Its disadvantage is that the radiation source, as opposed to X-ray tubes, cannot be “turned off”, thus it is important to provide constant radiation protection.
The original document is available at http://537999.nhjqzg.asia/tiki-index.php?page=Radioactive+Isotopes